

Since Z = pv/RT, the specific volume at state 1 can be determined as follows:

SOLUTIONKnown: Water vapor is cooled at constant volume from 20 MPa, 520C to 400C.Find : Use the compressibility chart to determine the specific volume and final pressure and compare the results.(a) From Table Critical Constants : Using the compressibility chart, determinethe specific volume of the water vapor in m3/kg at the initial state.the pressure in MPa at the final state. SinceA closed, rigid tank filled with water vapor, initially at 20 MPa, 520C, is cooled until its temperature reaches 400C. The system completes 200 cycles/minNow, dE = 0, since cyclic integral of any property is zero. Sum of all heat transferred during the cycle = 340 kJ.Number of cycles completed by the system = 200 cycles/min. Complete the following table showing the method for each item, and compute the net rate of work output in kW. The sum of all heat transferred during a cycle is 340 kJ. Solution.Temperature rise, (T2 T1) = 25CThe heat transferred in the process, Q = 30 kJSpecific heat at constant volume, Cv = 1.2 kJ/kgCMass of the substance, m= 2.5 kgĪ fluid system, contained in a piston and cylinder machine, passes through a complete cycle of four processes. Determine: (i)The change in internal energy (ii)The work done The specific heat at constant volume for the pure substance comprising the system is 1.2 kJ/kgC, and the system contains 2.5 kg of this substance. The heat transferred in the process is 30 kJ. A closed system of constant volume experiences a temperature rise of 25C when a certain process occurs. Hence, heat rejected during the process = 3 kJ. Mass of nitrogen, m = 0.3 kgTemperature before compression = 40C or 313 KTemperature after compression = 160C or 433 KThe work done during the compression process, W = 30 kJAccording to first law of thermodynamics : Calculate the heat transferred from the nitrogen to the surroundings.Cv for N2= 0.75 kJ/kg K.Solution. The work done during the process is 30 kJ. The piston is moved compressing nitrogen until the pressure becomes 1 MPa and temperature becomes 160C. Work done by the fluid from 1 to 2 = Area 12 ML1Ġ.3 kg of nitrogen gas at 100 kPa and 40C is contained in a cylinder.
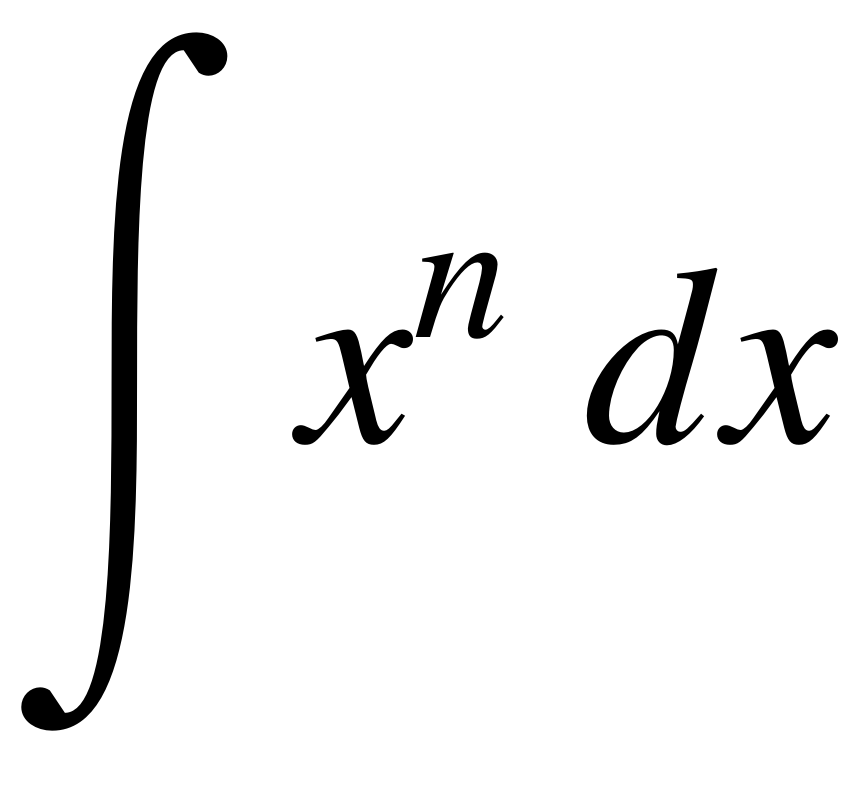
Calculate the net work done by the fluid, for an initial volume of 0.05 m3 The fluid is then cooled reversibly at constant pressure until the piston regains its original position heat is then supplied reversibly with the piston firmly locked in position until the pressure rises to the original value of 20 bar. The fluid is allowed to expand reversibly behind a piston according to a law pV2 = C until the volume is doubled. (b) Mean specific heat of the gas, cn:(a) Heat transferred, Q :A cylinder contains 1 kg of a certain fluid at an initial pressure of 20 bar.

Change in temperature of the gas = 25C to 125C The specific heat capacity of the system during a certain process is given by : If the mass of the gas is 6 kg and its temperature changes from 25C to 125C find : a. Find the absolute pressure of steam.Solution. The atmospheric pressure is 760 mm of Hg. Some of steam in the pipe condenses in the manometer arm connected to the pipe. The level of mercury in open arm is 97.5 mm greater than that in the arm connected to the pipe. TERMODINAMIKA TEKNIK KIMIA IContoh-contoh SoalDisusun oleh :Yelmida AA U-tube mercury manometer with one arm open to atmosphere is used to measure pressure in a steam pipe.


 0 kommentar(er)
0 kommentar(er)
